Vitamin C Autophagy
Metrics: Total Views: 0 (Spandidos Publications: | PMC Statistics: )
Metrics: Total PDF Downloads: 0 (Spandidos Publications: | PMC Statistics: )
This article is mentioned in:
It has been demonstrated that vitamin C exhibits anti-cancer activity in various tumor cell lines; however, its specific mechanism of action remains unknown. Although the diagnosis and therapy of cancer patients have markedly improved in recent years, safer and more cost‑effective treatments are still required. Therefore, the present study examined the effect of vitamin C on the induction of cell death in gastric cancer and its underlying mechanism of action. It was observed that the cytotoxicity of vitamin C on the human gastric cancer cell line AGS is dependent on the apoptotic pathway, including caspase cascades, but not on the necroptotic pathway. It was demonstrated that the vitamin C‑induced calcium influx and ROS generation have critical roles in the induction of apoptosis. Furthermore, vitamin C treatment depleted adenosine triphosphate (ATP) production in AGS cells, and the autophagy pathway may be involved in this process. Taken together, the current study suggests that a high dose of vitamin C may induce gastric cancer cell apoptosis through the dysfunction of mitochondria, including calcium influx, reactive oxygen species generation and ATP depletion.
Introduction
Gastric cancer is a complex and heterogeneous disease and one of the most frequently diagnosed types of cancer in Korea (1). Multiple unknown factors contribute to its pathogenesis, progression, metastasis and relapse (1,2). Currently, the main treatment options for gastric cancer are surgery, radiation therapy and chemotherapy (1,2). There have been numerous improvements in the diagnosis and treatment of gastric cancer (2). However, the development of novel therapeutic strategies is still required to reduce the debilitating side effects of the drugs currently used in chemotherapy and radiotherapy (2).
The majority of mammals are able to synthesize their own vitamin C in the liver by the action of the enzyme L-gulonolactone oxidase (3). However, certain primates, including humans, cannot produce vitamin C themselves due to a mutation in the gene encoding L-gulonolactone oxidase (3). It has been demonstrated that vitamin C has various beneficial effects, including anti-inflammatory and anti-oxidative activity (4). In cancer therapy, however, vitamin C exhibits pro-oxidant activity that often enhances its cytotoxic effects, including cellular damage through the accumulation of hydrogen peroxide (H2O2), which leads to the arrest of tumor cell growth and the induction of tumor cell death (4).
Apoptosis and necrosis are the two major mechanisms of cell death (5,6). Apoptosis is a method of programmed cell death that under normal conditions occurs as a homeostatic mechanism to maintain the population of cells in tissues and as a defense mechanism to remove cells damaged by noxious agents or disease (5,6). The apoptotic process is initiated by recognizing the death signal either from outside the cell or from mitochondria within the cell, leading to the activation of various caspases that results in DNA/RNA fragmentation, nuclear chromatin condensation, protein degradation, cell shrinkage and ultimately the shedding of apoptotic bodies (5,6). Phagocytic cells remove the released apoptotic bodies by engulfment without inflammation (5,6). By contrast, necrosis is a non-programmed mechanism of cell death initiated by a high level of toxic materials (5,6). The necrotic process begins with the cell swelling instead of shrinking and is accompanied by the formation of large vacuoles (5,6). It ends with complete cell lysis and diffusion of disrupted intracellular contents, eliciting inflammatory responses in the adjacent tissue (5,6). In addition, as an alternative form of programmed cell death, necroptosis is a form of regulated necrosis induced by signals received by death receptors, including tumor necrosis factor receptor (TNFR) 1, TNFR2 and FASR, or pattern recognition receptors (6). Necrosis and necroptosis are typically not associated with caspase activation (6).
A previous report demonstrated that vitamin C produces H2O2, thus inducing the apoptosis of human adenocarcinoma gastric cancer cells from the AGS cell line (7). A concentration of vitamin C >1 mM can produce H2O2, causing the death of cancer cells by producing the ascorbate radical (4). However, H2O2 generally causes cell swelling, which does not usually occur during necrosis (8). Therefore, it is necessary to examine whether reactive oxygen species (ROS) production induced by vitamin C mediates the apoptosis or necrosis of AGS cells. The present study aimed to confirm that a high concentration of vitamin C induces cell death in the AGS cell line and to determine whether vitamin C-induced cell death is triggered by apoptosis or necrosis.
Materials and methods
Cell line and cytotoxic assays
The human gastric cancer cell line AGS was purchased from the Korean Cell Line Bank (Cancer Research Institute, Seoul National University of Medicine, Seoul, Korea). AGS cells were grown in RPMI 1640 medium (Lonza, Walkersville, MD, USA) supplemented with 5% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and a 1% penicillin-streptomycin mixture (Gibco; Thermo Fisher Scientific, Inc.). Cells were incubated at 37°C in 5% CO2. A total of 7×104 AGS cells were plated in RPMI 1640 medium for 24 h in a 24-well plate. Cells were subsequently treated with incremental doses (0, 0.5 and 1.5 mM) of vitamin C (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 4 or 24 h. Cell viability was determined by a colorimetric method using 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) as described previously (9).
Analysis of cell death mechanism (apoptosis vs. necroptosis)
To determine which mechanism is induced by vitamin C and causes the death of AGS cells, an inhibitor of caspase-dependent apoptosis, z-VAD-FMK (z-VAD; Tocris Bioscience, Bristol, UK) and a necroptosis inhibitor, necrostatin-1 (Nec-1; BioVision, Inc., Milpitas, CA, USA) were used. A total of 7×104 AGS cells were cultured in RPMI 1640 medium for 24 h in a 24-well plate. AGS cells were divided into four groups. Two groups were treated with either 0 or 1.5 mM vitamin C for 4 h, while the other two groups were pretreated with 20 mM nec-1 or 10 mM z-VAD for 1 h prior to co-incubation with vitamin C. Cell viability was determined by MTT assay.
Analysis of apoptosis with annexin V-propidium iodide (PI)
Apoptosis was quantified using PI (MBL International Co., Woburn, MA, USA) and an Annexin V-FITC Apoptosis Detection kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer's protocol. Briefly, following centrifugation at 400 × g for 5 min at room temperature, the cells were double stained with annexin V-fluorescein isothiocyanate (FITC) and PI as recommended by the manufacturer. The apoptotic cell population was determined using a FC 500 flow cytometry system (BD Biosciences) and analyzed with CXP 2.2 Acquisition Software CXP 2.2 Analysis Software (Beckman Coulter, Inc., Brea, CA, USA). For each sample, ≥10,000 cells were measured.
Measurement of calcium
The level of intracellular calcium was measured using the fluorescent calcium indicator Fluo-3 (Invitrogen; Thermo Fisher Scientific, Inc.), as described previously (10). Briefly, cells were incubated with 1 µM Fluo-3 calcium indicator for 1 h. The cell intracellular calcium distributions were determined by using a FACSCalibur™ flow cytometer (BD Biosciences) and analyzed using the CellQuest Pro software program (BD Biosciences). For each sample, ≥10,000 cells were analyzed. To examine the role of intracellular calcium in the cytotoxicity and apoptosis induced by vitamin C, 10 µM calcium inhibitor 1,2-bis (2-aminophenoxy) ethane N,N,N',N'-tetraacetic acid (BAPTA; Tocris Bioscience) was incubated prior to co-incubation with vitamin C at the indicated concentrations. Cytotoxicity was determined by MTT assays, and apoptosis was analyzed by flow cytometry following double staining with annexin V-FITC and PI.
Measurement of ROS
The level of intracellular ROS was measured using a ROS detection reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's guidelines. ROS levels were measured with a FACSCalibur™ flow cytometer. To examine the role of intracellular ROS in the cytotoxicity and apoptosis induced by vitamin C, 5 mM antioxidant N-acetylcysteine (NAC; Tocris Bioscience) was incubated prior to co-incubation with vitamin C. Cytotoxicity was determined by MTT assays, and apoptosis was analyzed by flow cytometry following double staining with annexin V-FITC and PI.
Measurement of adenosine triphosphate (ATP)
The production of ATP was measured using the ATP Colorimetric/Fluorometric Assay kit (BioVision, Inc.) according to the manufacturer's guidelines.
Western blot analysis
AGS cells were cultured in 6-well plates and incubated with vitamin C at different concentrations for 4 h. Following incubation, cells were washed with ice-cold phosphate-buffered saline and lysed with lysis buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS) and 1% NP-40] containing a protease inhibitor cocktail (Calbiochem; Merck Millipore). Cell debris was removed by centrifugation at 18,210 × g for 30 min, and protein concentration was determined using a Bradford Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to an Immobilon-P nitrocellulose membrane (0.45 µm; Merck Millipore) using the TE 77 Semi-Dry Transfer Unit (GE Healthcare Life Sciences, Chalfont, UK). The membrane was blocked with 5% non-fat milk in Tris-buffered saline containing 1% Tween-20 (pH 7.4) at room temperature for 1 h, and blots were probed with rabbit monoclonal antibodies for pro-caspase-3 (1:200 dilution; Cat# 7148; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), cleaved caspase-3 (1:200 dilution; Cat# 22171-R; Santa Cruz Biotechnology, Inc.) and light chain 3 (LC3) I and LC3 II (1:3,000 dilution; Cat# 51520; Abcam, Cambridge, UK), or with mouse monoclonal antibody for β-actin (1:5,000 dilution; Cat# 47778; Santa Cruz Biotechnology, Inc.) at 4°C overnight, and then incubated at room temperature for 1 h with goat anti-rabbit or anti-mouse monoclonal antibodies (1:5,000 and 1:10,000 dilution, respectively; Cat# 2004 and 2055, respectively; Santa Cruz Biotechnology, Inc.). The proteins were visualized using an enhanced chemiluminescence kit and western blotting detection reagents (GE Healthcare Life Sciences, Pittsburgh, PA, USA), followed by exposure to X-ray film (Fujifilm, Tokyo, Japan). Each band was determined quantitatively using ImageJ software (http://rsb.info.nih.gov). The densitometry reading of the bands was normalized to β-actin expression.
Statistics
Statistical analyses were performed using GraphPad Prism version 5.03 software (GraphPad Software, Inc., La Jolla, CA, USA). Differences among groups were analyzed using a two-tailed t-test. P<0.05 was considered to indicate a statistically significant difference. Data were represented as the mean ± standard deviation.
Results
Vitamin C decreases the viability of human gastric cancer cells
To examine whether treatment of high-dose vitamin C affects the growth of AGS cells, cells were treated with various concentrations of vitamin C for 4 or 24 h, and their cytotoxicity was subsequently analyzed using an MTT assay. Vitamin C treatment decreased the number of AGS cells in a dose-dependent manner (Fig. 1). Although a longer incubation time slightly increased the susceptibility to cell death induced by vitamin C treatment, there was no significant difference between cell viability at 4 and 24 h, except when cells were incubated with 0.5 mM vitamin C. The half maximal effective concentration (EC50) values of the cells incubated for 4 and 24 h were 1.2 and 1.1 mM, respectively (Fig. 1). This result indicates that high-dose vitamin C has an anti-tumor effect in the AGS cell line.
Vitamin C-induced cell death is dependent on apoptosis, not necroptosis
Apoptosis and necrosis (or the form of necrosis known as necroptosis) may occur simultaneously depending on certain factors, including stimulus intensity and duration, caspase availability and the extent of ATP depletion (5). To examine whether the death of AGS cells by vitamin C was dependent on apoptosis or necroptosis, AGS cells were incubated with an apoptosis-specific inhibitor or necroptosis-specific inhibitor prior to vitamin C treatment. Pretreatment with z-VAD significantly inhibited vitamin C-induced cell death, regardless of vitamin C concentration (Fig. 2). By contrast, pretreatment with Nec-1 enhanced the reduction in cell viability induced by vitamin C (Fig. 2). This unexpected decrease is consistent with the results of a previous study demonstrating that Nec-1 treatment strongly inhibits programmed cellular necrosis while somewhat increasing apoptotic cell death (11). This result suggests that apoptosis, not necrosis, primarily mediates the AGS cell death that occurs following treatment with high-dose vitamin C.
Increased intracellular calcium is partially responsible for AGS cell apoptosis induced by vitamin C
Several previous studies have reported that releasing calcium from the endoplasmic reticulum (ER) into the cytosol induces apoptosis in cancer cells (12–14). The present study demonstrated that treatment of AGS cells with 0.5 and 1.5 mM vitamin C for 4 h significantly increased the amount of intracellular calcium, by 5.78 and 36.00%, respectively (Fig. 3A and B). To examine whether the increased calcium concentration induced by vitamin C affects the viability of AGS cells, cells were treated with 1.5 mM vitamin C following pretreatment with BAPTA. The inhibition of calcium accumulation by BAPTA pretreatment significantly suppressed the cytotoxic activity of vitamin C (Fig. 3C). To confirm the effect of calcium accumulation on vitamin C-induced apoptosis, AGS cells treated in the same way were stained with annexin V and PI, and subsequently analyzed by flow cytometry. Consistent with the cytotoxicity results, vitamin C markedly increased the rate of late apoptosis by 16.29%, whereas BAPTA pretreatment reduced vitamin C-induced late apoptosis by 11.99% (Fig. 3D and E). These data indicate that the accumulation of intracellular calcium, at least in part, contributes to the apoptotic effect of vitamin C in AGS cells.
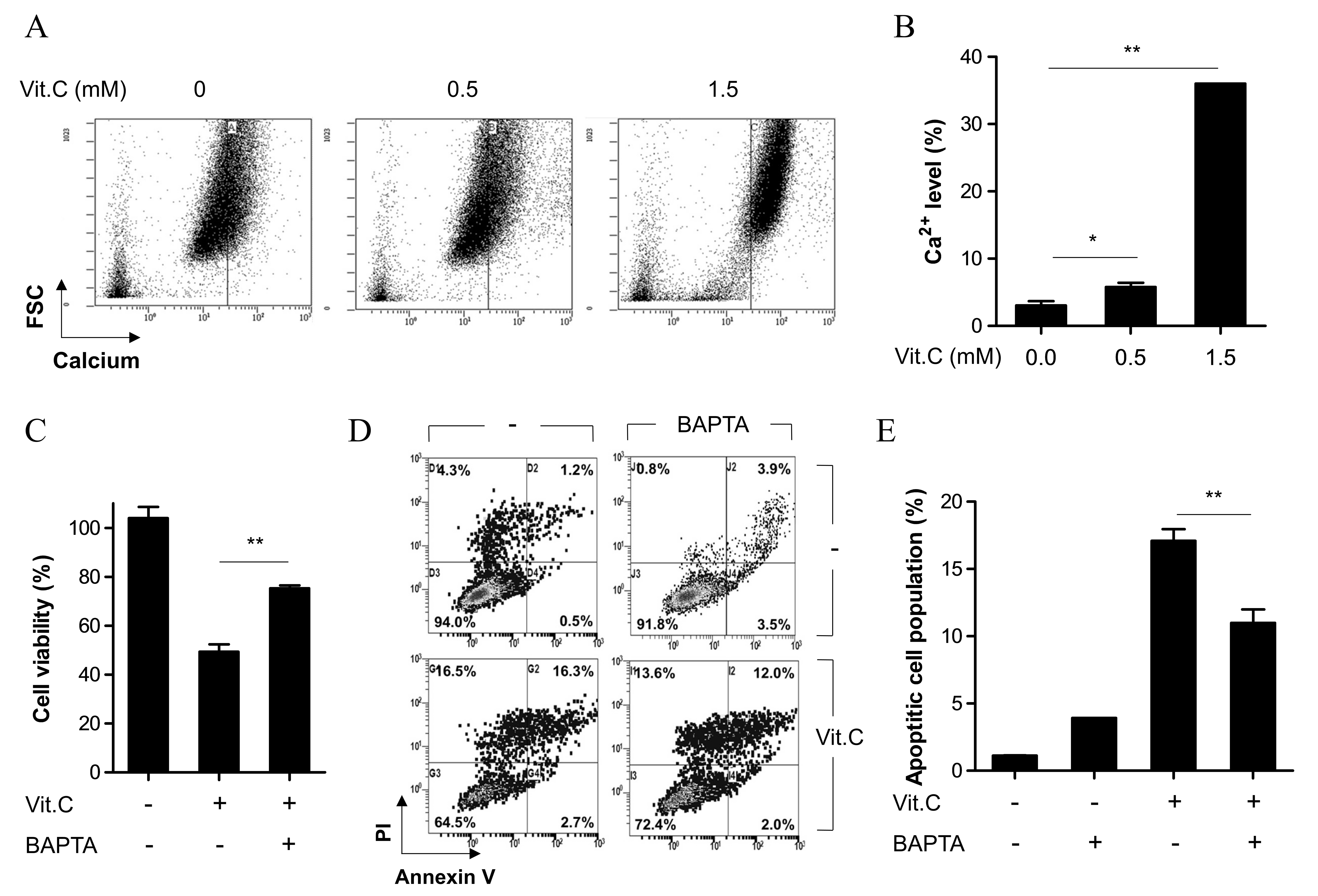 | Figure 3.Calcium concentrations increased by Vit. C serve a role in AGS cell apoptosis. AGS cells were treated with 0.5 or 1.5 mM Vit. C for 4 h. Cells were subsequently treated with Fluo-3, a fluorescent calcium indicator, and analyzed using flow cytometry. (A) Representative plots of intracellular calcium analysis are shown. (B) The calcium-positive cell population is shown as the mean ± standard deviation. AGS cells were pretreated with 10 µM BAPTA, and 1 h later, 1.5 mM Vit. C was added to the cells. Following 4 h of treatment with Vit. C, cell viability and apoptotic cell population were analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and by annexin V/PI staining, respectively. (C) Cell viability is shown as the mean ± standard deviation. (D) Representative plots of apoptotic cell analysis are shown. (E) The ratio of apoptotic cell population is shown as the mean ± standard deviation. *P<0.05, **P<0.01. Vit., vitamin; FSC, forward scatter; PI, propidium iodide; BAPTA, 1,2-bis (2-aminophenoxy) ethane N,N,N',N'-tetraacetic acid. |
Enhanced generation of ROS is indispensable for AGS cell apoptosis induced by vitamin C
Several studies have reported that vitamin C has numerous anti-oxidative activities, whereas other studies argue its pro-oxidative properties (4,15,16). To determine whether the apoptotic activity of vitamin C in the AGS cells is associated with its anti-oxidant or pro-oxidant properties, the amount of ROS in the cells was measured. When AGS cells were treated with 0.5 and 1.5 mM vitamin C, ROS levels increased by 13.56 and 22.97%, respectively (Fig. 4A and B). To examine whether enhanced ROS generation contributes to the cytotoxic activity of vitamin C, AGS cells were pretreated with the antioxidant NAC prior to the addition of vitamin C. NAC pretreatment significantly reduced the rate of vitamin C-induced cell death (Fig. 4C). In addition, NAC pretreatment markedly suppressed the late apoptosis induced by vitamin C to 1.29% (Fig. 4D and F). These data suggest that the generation of intracellular ROS by vitamin C treatment is involved in the induction of apoptosis. Therefore, the apoptotic activity of vitamin C is associated with its pro-oxidant properties.
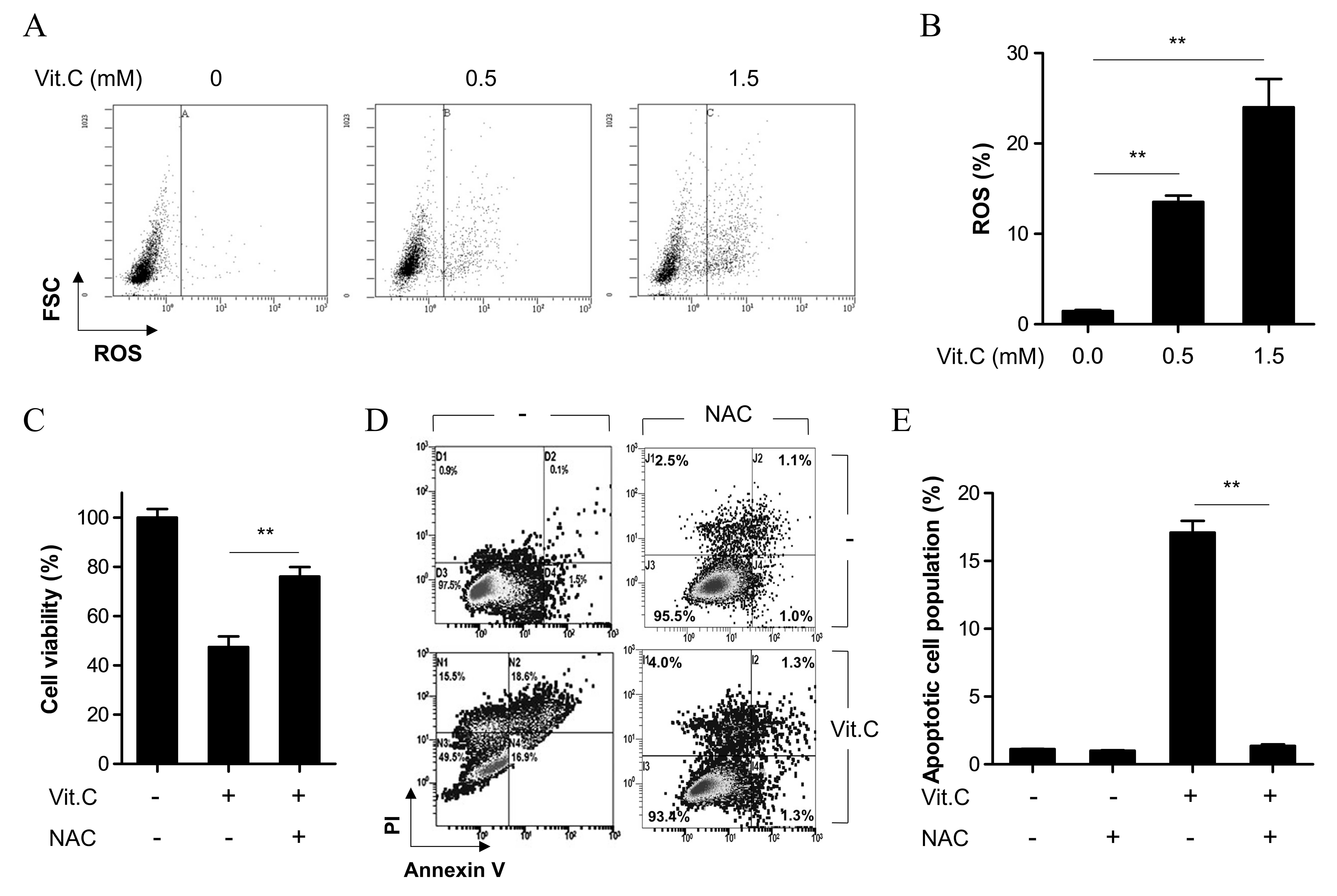 | Figure 4.Vit. C increases the levels of ROS, which is critical in the apoptosis of AGS cells. AGS cells were treated with 0.5 or 1.5 mM Vit. C for 4 h and the levels of intracellular ROS were analyzed using a ROS detection kit and flow cytometry. (A) Representative plots of intracellular ROS analysis are shown. (B) The ratio of ROS-positive cell population is shown as the mean ± standard deviation. AGS cells were pretreated with 5 mM NAC, and 1 h later, 1.5 mM Vit. C was added to the cells. Following 4 h of Vit. C treatment, the cell viability and apoptotic cell population were analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and by annexin V/PI staining, respectively. (C) The cell viability is shown as the mean ± standard deviation. (D) Representative plots of apoptotic cell analysis are shown. (E) The ratio of apoptotic cell population is shown as the mean ± standard deviation. **P<0.01. PI, propidium iodide; Vit., vitamin; FSC, forward scatter; ROS, reactive oxygen species; NAC, N-acetylcysteine. |
Apoptosis, autophagy and mitochondrial dysfunction are involved in the cell death process induced by vitamin C
To identify the molecular mechanisms underlying vitamin C-induced cell death, the expression of pro-caspase-3 and its cleaved form, which are indicators for apoptosis (5), as well as that of two types of the microtubule-associated protein LC3 (LC3 I and LC3 II), which are indicators for the autophagy pathway (17), were assessed. Although there were no differences in the expression of any of the above proteins at low doses of vitamin C, higher concentrations of vitamin C increased the cleavage of caspase-3 and increased LC3 II expression (Fig. 5A and B). These results support that apoptosis may be the primary mechanism by which vitamin C-induced cell death occurs, and that autophagy may be associated with the apoptosis pathway.
Figs. 3 and 4 demonstrate that vitamin C modulated certain factors associated with the mitochondrial function, including intracellular Ca2+ and ROS (18). Therefore, other mitochondrial function, ATP production (18), was additionally examined. ATP generation in the cells was markedly decreased by vitamin C in a dose-dependent manner (Fig. 6), indicating that vitamin C may cause overall mitochondrial dysfunction as well as apoptosis induction.
Discussion
The clinical efficacy of vitamin C treatment in patients with cancer is controversial (4). However, several studies have reported that plasma concentrations of vitamin C in humans following high-dose intravenous injection are 5–15 mM, while those achieved following oral administration are limited to 0.15–0.20 mM (4,19,20). In the majority of cancer cell lines, concentrations of vitamin C <5 mM cause 50% cell death, however concentrations >20 mM are nontoxic in normal cells (4). Notably, in the present study, the EC50 of vitamin C in AGC cells was 1.0–1.3 mM. These values have important clinical implications, as the concentrations are in the range achieved following intravenous injection of high-dose vitamin C (4). In addition, it was demonstrated that intravenous injection of vitamin C has remarkably few side effects in a study of >20,000 patients over 2 years (20). Therefore, the use of high-dose vitamin C treatment in cancer therapy should be re-evaluated.
The ER and mitochondria are important intracellular organelles in the TNFR-independent apoptosis pathway (14). Excess calcium that has escaped from the ER may enhance the production of ROS from mitochondria, and the increased mitochondrial generation of Ca2+ ions and ROS synergistically may lead to the alteration of the mitochondrial permeability transition pore, resulting in cell death (13,14). In addition, previous studies have reported that a high dose of vitamin C increases the susceptibility of cancer cells to apoptosis by activating mitochondrial 14-3-3σ and 14-3-3β (21,22). In line with these reports, the present study demonstrated that a high dose of vitamin C increases the levels of intracellular Ca2+ and ROS, and decreases the production of intracellular ATP, which is mainly produced by mitochondria. This indicates that mitochondrial dysfunction may be an important process in the vitamin C-induced cell death of AGS cells.
Furthermore, the results from the current study indicated that the expression of the autophagy indicator protein LC3 II was markedly increased following treatment with high-dose vitamin C. It has been demonstrated that autophagy is involved in the turnover of unnecessary proteins and whole organelles, and is therefore predominantly a cytoprotective process to help maintain the healthy condition of normal cells (6). However, excessive activation of autophagy under certain circumstances is linked to mechanisms of cell death (6). Thus, the vitamin C-induced autophagy in the present study may be linked to apoptosis, but not to necrosis or cytoprotective processes. Future studies are required to examine the differences between autophagy induction in healthy cells and in those treated with vitamin C.
In conclusion, the present study demonstrated that vitamin C is able to induce apoptosis in human gastric cancer cells in a caspase-dependent manner. This process involves mitochondrial dysregulation via intracellular Ca2+ efflux from ER, ROS generation from mitochondria and decreased levels of ATP. Additionally, the effective dose of vitamin C for inducing apoptotic cell death in humans is achievable by intravenous injection of high-dose vitamin C. Therefore, high-dose vitamin C treatment may be developed in the future as a novel effective therapeutic strategy for patients with gastric cancer.
Acknowledgements
The present study was supported by a grant from the National Research and Development Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (Seoul, Korea; grant no. 0820050).
References
| 1 | Shin A, Kim J and Park S: Gastric cancer epidemiology in Korea. J Gastric Cancer. 11:135–140. 2011. View Article : Google Scholar : PubMed/NCBI |
| 2 | Lordick F, Allum W, Carneiro F, Mitry E, Tabernero J, Tan P, Van Cutsem E, van de Velde C and Cervantes A: Unmet needs and challenges in gastric cancer: The way forward. Cancer Treat Rev. 40:692–700. 2014. View Article : Google Scholar : PubMed/NCBI |
| 3 | Levine M, Dhariwal KR, Welch RW, Wang Y and Park JB: Determination of optimal vitamin C requirements in humans. Am J Clin Nutr. 62:(6 Suppl). 1347S–1356S. 1995.PubMed/NCBI |
| 4 | Ohno S, Ohno Y, Suzuki N, Soma G and Inoue M: High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 29:809–815. 2009.PubMed/NCBI |
| 5 | Elmore S: Apoptosis: A review of programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI |
| 6 | Nikoletopoulou V, Markaki M, Palikaras K and Tavernarakis N: Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013. View Article : Google Scholar : PubMed/NCBI |
| 7 | Ha YM, Park MK, Kim HJ, Seo HG, Lee JH and Chang KC: High concentrations of ascorbic acid induces apoptosis of human gastric cancer cell by p38-MAP kinase-dependent up-regulation of transferrin receptor. Cancer Lett. 277:48–54. 2009. View Article : Google Scholar : PubMed/NCBI |
| 8 | Simon F, Varela D, Riveros A, Eguiguren AL and Stutzin A: Non-selective cation channels and oxidative stress-induced cell swelling. Biol Res. 35:215–222. 2002. View Article : Google Scholar : PubMed/NCBI |
| 9 | Lee GW, Park HS, Kim EJ, Cho YW, Kim GT, Mun YJ, Choi EJ, Lee JS, Han J and Kang D: Reduction of breast cancer cell migration via up-regulation of TASK-3 two-pore domain K+ channel. Acta Physiol (Oxf). 204:513–524. 2012. View Article : Google Scholar : PubMed/NCBI |
| 10 | Nakamura TY, Yamamoto I, Nishitani H, Matozaki T, Suzuki T, Wakabayashi S, Shigekawa M and Goshima K: Detachment of cultured cells from the substratum induced by the neutrophil-derived oxidant NH2Cl: Synergistic role of phosphotyrosine and intracellular Ca2+ concentration. J Cell Biol. 131:509–524. 1995. View Article : Google Scholar : PubMed/NCBI |
| 11 | Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB and Martin LJ: Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 31:178–189. 2011. View Article : Google Scholar : PubMed/NCBI |
| 12 | Pinton P, Giorgi C, Siviero R, Zecchini E and Rizzuto R: Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 27:6407–6418. 2008. View Article : Google Scholar : PubMed/NCBI |
| 13 | Lemasters JJ, Theruvath TP, Zhong Z and Nieminen AL: Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 1787:1395–1401. 2009. View Article : Google Scholar : PubMed/NCBI |
| 14 | Bravo-Sagua R, Rodriguez AE, Kuzmicic J, Gutierrez T, Lopez-Crisosto C, Quiroga C, Díaz-Elizondo J, Chiong M, Gillette TG, Rothermel BA and Lavandero S: Cell death and survival through the endoplasmic reticulum- mitochondrial axis. Curr Mol Med. 13:317–329. 2013. View Article : Google Scholar : PubMed/NCBI |
| 15 | Carr A and Frei B: Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 13:1007–1024. 1999.PubMed/NCBI |
| 16 | Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK and Levine M: Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J Am Coll Nutr. 22:18–35. 2003. View Article : Google Scholar : PubMed/NCBI |
| 17 | Mizushima N and Yoshimori T: How to interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007. View Article : Google Scholar : PubMed/NCBI |
| 18 | Csordás G and Hajnóczky G: SR/ER-mitochondrial local communication: Calcium and ROS. Biochim Biophys Acta. 1787:1352–1362. 2009. View Article : Google Scholar : PubMed/NCBI |
| 19 | Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J and Cantilena LR: Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 93:3704–3709. 1996. View Article : Google Scholar : PubMed/NCBI |
| 20 | Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J and Levine M: Vitamin C: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PloS One. 5:e114142010. View Article : Google Scholar : PubMed/NCBI |
| 21 | Nagappan A, Park KI, Park HS, Kim JA, Hong GE, Kang SR, Lee DH, Kim EH, Lee WS, Won CK and Kim GS: Vitamin C induces apoptosis in AGS cells by down-regulation of 14-3-3σ via a mitochondrial dependent pathway. Food Chem. 135:1920–1928. 2012. View Article : Google Scholar : PubMed/NCBI |
| 22 | Kim JE, Kang JS and Lee WJ: Vitamin C induces apoptosis in human colon cancer cell line, HCT-8 via the modulation of calcium influx in endoplasmic reticulum and the dissociation of bad from 14-3-3beta. Immune Netw. 12:189–195. 2012. View Article : Google Scholar : PubMed/NCBI |
Source: https://www.spandidos-publications.com/10.3892/ol.2016.5212

Tidak ada komentar: